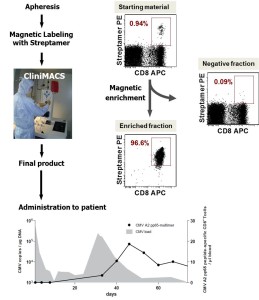
Isolation of virus- and tumor-specific T cells for clinical translation
The overall aim of the project is to provide all preclinical data and processes necessary to file an “investigation medicinal product” application for phase I/II trials employing tumorantigen (WT-1)-specific donor lymphocytes in oncologic patients. Therefore, we are aiming at establishing and validating GMP-compliant processes for the isolation and release testing of highly purified virus- and tumorantigen (Wilms Tumor (WT)-1)-specific donor lymphocytes. This will not only involve the isolation of WT-1 antigen specific donor lymphocytes using MHC class I-peptide Streptamer-technology at clinical grade, but also the evaluation and validation of protocols that will allow a cryopreservation of the isolated CD8+ cytotoxic T-Lymphocytes. Based on the optimised protocols a manufacturing license according to §13 AMG will be granted with the federal state authority, which is the prerequisite to apply for the phase I/II trials according to §42 AMG with the Paul-Ehrlich-Institute.